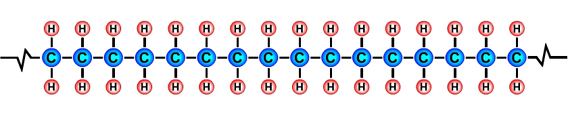
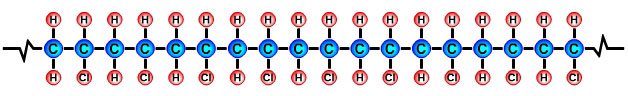
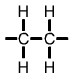
Formulae of common polymersHydrocarbons obtained from fossil fuels are broken down into simpler molecules in the "cracking process". The cracking process involves heating a hydrocarbon in the presence of a catalyst which causes it to break down into simpler molecules such as ethylene (ethene) C2H4, propylene (propene) C3H6, and butene C4H8. A single unit of a molecule like ethene is called a monomer. Polymers are created by linking lots of units of a monomer in a long chain in a process called polymerisation. Single units (monomers) of the more common polymers are shown below. |
||
 |
 |
 |
Polyethylene (PE) monomer |
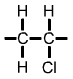
Polyvinyl Chloride (PVC) monomer |
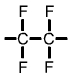
Polytetrafluoroethylene (PTFE) monomer |
|
||
The PVC polymer formula is similar to the PE polymer formula but you will notice |
||
 |
 |
 |
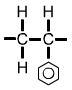
Polystyrene (PS) monomer |
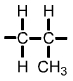
Polypropylene (PP) monomer |
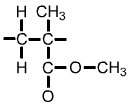
Polymethyl methacrylate (PMMA) monomer |
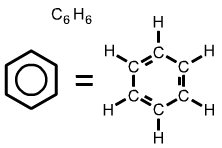
The formula for polystyrene (shown above) includes a hexagon with a circle inside. The hexagonal symbol represents the compound benzene, C6H6. |
|
|
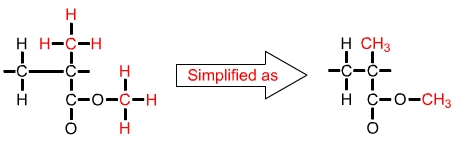
The formula for a single unit of polymethyl methacrylate (PMMA) is C5O2H8. This can be shown as in the diagram above left, or simplified as in the diagram above right. |
||