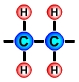
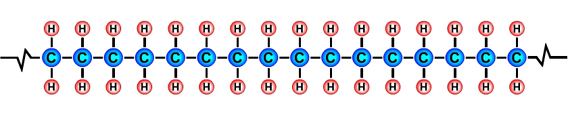
PlasticThe term plastic means that a material is soft and can be shaped and moulded. The materials that we call plastics, e.g. acrylic, polythene and nylon, have at some point been soft and have been shaped and moulded. The term polymer comes from the Greek words "poly" meaning many and "mer" meaning units. Polymers are made up of lots of units of molecules (monomers) strung together in a tangled mass. The repeating units of monomers usually have a carbon backbone, e.g. the polyethylene molecule, illustrated below.
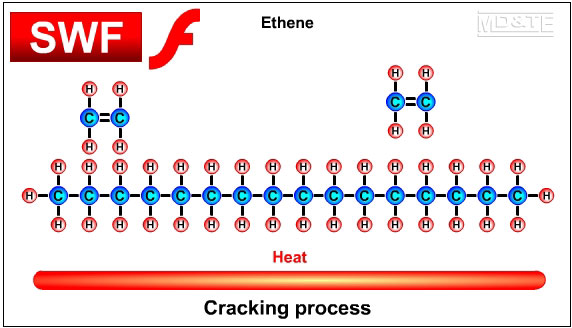
The main ingredients of polymersThe main ingredients of many polymers are the elements hydrogen and carbon. Carbon forms the backbone of most polymers. (See the diagram of the polyethylene molecule above). The hydrocarbons used to create plastics are most often obtained from the fossil fuels crude oil, coal and natural gas, however bioplastics are being developed from hydrocarbons that are obtained from natural organic materials such as plants and algae. Bioplastics have the advantages of being biodegradable and sustainable. Other elements that are often combined with hydrocarbons to create polymers include oxygen, nitrogen, fluorine, chlorine, sulphur, phosphorous and silicon. Creating monomers: the "cracking process"A hydrocarbon may be broken down into simpler molecules by a "cracking process." The cracking process involves heating a hydrocarbon in the presence of a catalyst which causes it to break down into simpler molecules such as ethylene (ethene) C2H4, propylene (propene) C3H6, and butene C4H8. A single unit of a molecule like ethene is called a monomer. In the example below, the long chain molecule C16H34 is broken down into the simpler molecules Ethene
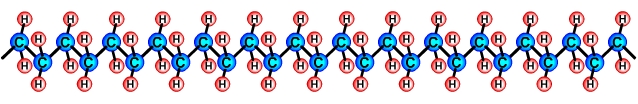
Creating polymersPolymers are created by linking lots of units of a monomer in a long chain in a process called polymerisation. For example, a polymer called Polyethylene, also called Polythene is made by linking lots of units of the Ethylene C2H4 monomer in a long chain. Polymer chains are usually drawn as 2 dimensional objects for simplicity (see above), however they are long 3 dimensional structures, (see below).
The physical, chemical and visual properties of a polymer are determined by the length of the chain, the elements that make up the polymer and the way that the elements are combined and structured. (Click to see the formulae of commonly used polymers.) The properties of polymers may be modified to make them more suitable for special purposes:
AdditivesAdditives are chemicals added to polymers. Additives such as initiators, antioxidants, foaming agents, plasticisers and colourants are combined with polymers to initiate cross linking and to enhance the physical, chemical or visual properties of polymers. For example, additives can:
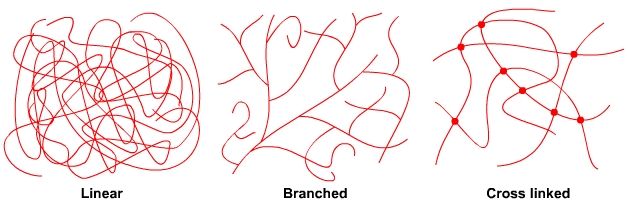
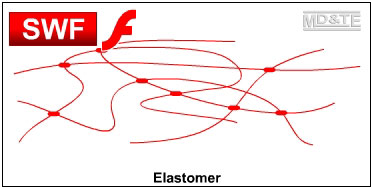
ThermoplasticsThermoplastic polymers are either long string-like lines of molecules or long string-like lines of molecules with side branches. The molecules are in a tangled mass that resembles spaghetti. The polymer chains are held together by weak forces between the molecules, called van der Waals forces and by the entanglement of the chains. There are no bonds between the lines of polymer chains. When a thermoplastic is heated, the forces between the polymer chains weaken and the polymer softens and then melts. In the softened state, thermoplastics can be moulded and reshaped, e.g. by vacuum forming. A thermoplastic can be reheated and reshaped many times. A thermoplastic like acrylic will return to its original shape when reheated unless it is held in its deformed position. The plastic is said to have plastic memory. ThermosetsThermosetting polymers consist of long chains of molecules that form bonds between the chains during polymerisation. This forms a single network of tangled polymer chains. Once polymerisation is complete, the polymer chains are set and cannot be softened and reshaped. An uncured thermosetting polymer is in liquid or semi solid form and has no bonds between the chains of molecules. It may be softened by heating and may be shaped and formed under pressure. However, the heating triggers a chemical reaction that initiates cross linking between the polymer chains that permanently and irreversibly locks the polymer molecules together in a “set” arrangement. The chemical reaction causes the polymer to go hard and stay hard, similar to hard boiling an egg.
SMART polymersSMART polymers change in some way when exposed to a change in the environment. The change in the polymer may be a change in colour, a change in solubility, a change in size or a change in shape etc. The changes may be initiated by:
Examples of useTemperature sensitive polymers that change colour may be used for plastic cups that inform the user when the liquids inside are too hot or too cold to drink. Shape Memory Polymers, (SMPs) change shape when triggered by a change in temperature, an electric current, magnetic field, light or liquids making them useful for surgical implants, actuators and switches. Another exciting medical use of smart polymers is the development of bioplastic delivery systems that release medications over a period of time, e.g. a delivery system based on a polylactic acid (PLA) based, phase-sensitive polymer. |
||||||||
| Click here to view the PDF version of this resource. | 
|
 |
||||||